HeaRTNovation
HEMOSTAT: Hemostatic Agents for Wounds or Skin Openings
Technology Generator
Department of Science and Technology-Philippine Nuclear Research Institute (DOST-PNRI)
Project leader: Ms. Charito Aranilla
The Problem
Hemorrhage or bleeding is a condition that occurs when blood vessels are damaged. Its severity may range from minor cuts to life-threatening traumatic wounds. If left untreated, severe hemorrhage may lead to trauma and even death.
Controlling the bleeding to prevent further blood loss is critical for the survival of the patients. Antihemorrhagic agents are materials capable of inducing rapid clotting of the wound. These are used to control heavy bleeding in medical procedures, military operations, and emergency cases. There are different types of antihemorrhagic agents in the market, such as fibrin sealants, bovine gelatin, oxidized cellulose, and chitosan. These agents, however, are only available overseas and are expensive. Prices range from PHP 1,000 to PHP 6,000 for single use packs.
The Solution
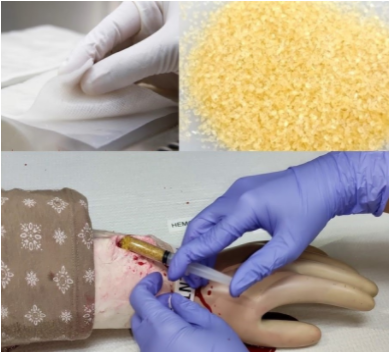
The Department of Science and Technology-Philippine Nuclear Research Institute (DOST-PNRI), through a research led by Ms. Charito Aranilla, and funded by the Philippine Council for Health Research and Development (PCHRD), developed an efficient, inexpensive, and safe hemostatic agent from locally available raw materials. Using PNRI’s radiation technology, the researchers successfully developed two types of hemostatic materials, granules and dressing, with high coagulation efficiency.
Based on laboratory studies, the Hemostat granules and dressings significantly controlled bleeding caused by punctured vessels and deep wounds in animal models. The granules form a gel-network on the bleeding site that is capable of adjusting to the size of the wound. The granules are tightly bonded, preventing it from being easily removed but can simply be washed off by water. The dressing forms a cotton network filled with gel formulation initiating the blood clotting activity on the wounds.
Product Development Stage
The Hemostat technology is at Technology Readiness Level (TRL) 4. A patent application is already filed locally and the technology will undergo clinical trials in collaboration with potential adopters.
Contact person
Mr. Gregory R. Ciocson
Business Development Specialist
Department of Science and Technology-Philippine Nuclear Research Institute
Commonwealth Avenue, Diliman,
Quezon City, Metro Manila, 1101, Philippines
Telephone line: (02) 8929 6011
Email address: gciocson.pnri@gmail.com
Website: https://www.pnri.dost.gov.ph/




