HeaRTNovation
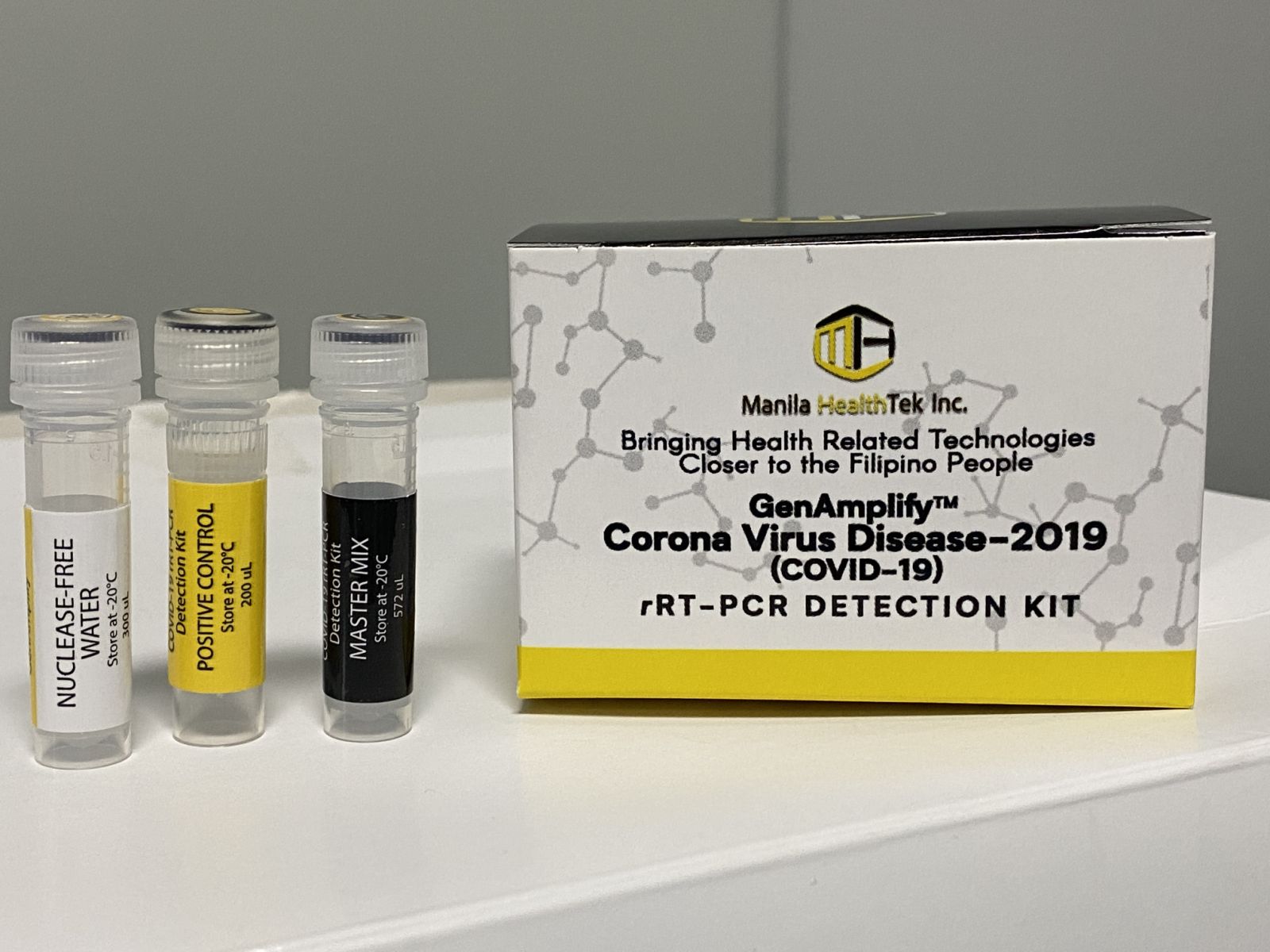
GenAmplify™ COVID-19 rT-PCR Detection Kit
Technology Generator
Manila HealthTek, Inc.
Project Leader: Ms. Joyce Ann Santos
The Problem
Coronavirus disease (COVID-19) is a global pandemic that has affected 150 million people and 220 countries to date. This is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The outbreak of COVID-19 was declared as a Public Health Emergency of International Concern (PHEIC) by the World Health Organization on 30 January 2020 due to its fast transmission across countries worldwide. It continues to leave casualties and the Philippines is no exception. COVID-19 cases in the country started to rise in March 2019 and there are already 1,054,983 confirmed cases as of 03 May 2021.
This surge in positive cases poses an immediate and strong need for a COVID-19 detection kit that is accurate, fast, and inexpensive. This will address the need for a reliable test with a shorter turnaround time for the results and is accessible to the general population so more people can be tested.
The Solution
A team of 15 scientists from the Philippine Genome Center, which includes the inventor of Biotek™ M Dengue Kit, Dr. Raul V. Destura, and the University of the Philippines Manila – National Institutes of Health (UPM-NIH) successfully developed a locally-made real-time reverse transcription polymerase chain reaction kit called GenAmplify™ Corona Virus Disease-2019 rRT-PCR Detection Kit through a research funded by the DOST-PCHRD. The detection kit tests for the presence of SARS-CoV-2 via nasopharyngeal and oropharyngeal swabs.
GenAmplify™ was developed through Next Generation Sequencing, a process that identifies the DNA and properties of several unknown organisms. It had undergone further verification processes to ensure its usefulness. It was tested in all bacteria and viruses that can be present in the lungs to make sure that the kit only yields a positive reaction to SARS-CoV-2. Further tests showed its capacity to amplify a very small size of the virus, up to 0.05 copies per one microliter, specifically.
To date, GenAmplify™ has already been rolled out to various hospitals all over the country. Since the kit is locally sourced, its production cost is relatively inexpensive hence, more supplies can be made, and delivery is faster making the kits more accessible to Filipinos who need to undergo COVID-19 testing.
Product Development Stage
GenAmplify™ Corona Virus 2019 (COVID-19) rRT-PCR Detection Kit is currently at Technology Readiness Level (TRL) 9, wherein the technology is available for consumers. The second version of the kit was given a certification of validity and reliability by the Food and Drug Administration last July 6, 2020 after passing confirmatory tests of Research Institute for Tropical Medicine (RITM) and DOST laboratories.
The first batch of the test kits is rolled out to various hospitals, both in and out of Metro Manila. Manila HealthTek, Inc. is responsible for the mass production and distribution of the test kits.
Contact Details
Manila HealthTek, Inc.
109 Mayor Gil Fernando Avenue,
Santa Elena, Marikina, Manila
talk2us@manila-healthtek.com




