HeaRTNovation
CleanIntubate: An Automated Laryngoscope Blade Disinfecting Device
Technology Generators
University of the Philippines Manila
University of the Philippines Diliman
Project leader: Catherine S. Co, MD
The Problem
 Laryngoscope blades are medical instruments used in intubation – a medical procedure performed on patients with respiratory compromise. The instrument is placed in the patient’s mouth to get a better view of the larynx and aid in the insertion of a tracheal tube. Since the instrument gets in contact with the patient’s mucous membranes, laryngoscope blades are classified as semi-critical equipment and thus require constant high-level disinfection.
Laryngoscope blades are medical instruments used in intubation – a medical procedure performed on patients with respiratory compromise. The instrument is placed in the patient’s mouth to get a better view of the larynx and aid in the insertion of a tracheal tube. Since the instrument gets in contact with the patient’s mucous membranes, laryngoscope blades are classified as semi-critical equipment and thus require constant high-level disinfection.
In the hospital setting, semi-critical medical equipment like the laryngoscope blades are commonly disinfected using washer-disinfectors. These are machines that allow the automated washing and disinfection of semi-critical equipment. This method, however, still requires a medical personnel to manually rinse and scrub the equipment until all visible soils are removed, then submerge the cleaned equipment in solutions of detergent and disinfectants, and finally dry the equipment before these are placed inside the washer-disinfector. Aside from being tedious and time-consuming, this manual preparation exposes the medical personnel to pathogens thereby increasing their risk of contracting infections.
Since the COVID-19 virus is transmitted via saliva droplets or mucous secretions, this further heightened the requirement for high-level disinfection of laryngoscope blades. Improper disinfection of laryngoscope blades may result in unwanted transmission of the said virus. There is therefore a need for a device that allows automated high-level disinfection of laryngoscope blades and at the same time lessens the manual handling of said contaminated equipment making the entire washing and disinfecting process fast but safe and effective.
The Solution
As part of their initiatives to strengthen the country’s fight against COVID-19, a group of medical professionals and engineers from the University of the Philippines Manila and the University of the Philippines Diliman, respectively, led by Dr. Catherine Co, developed a device called CleanIntubate. This device was generated under the UP Surgical Innovation and Biotechnology Laboratory (UP SIBOL) Program that is funded by the Philippine Council for Health Research and Development (PCHRD).
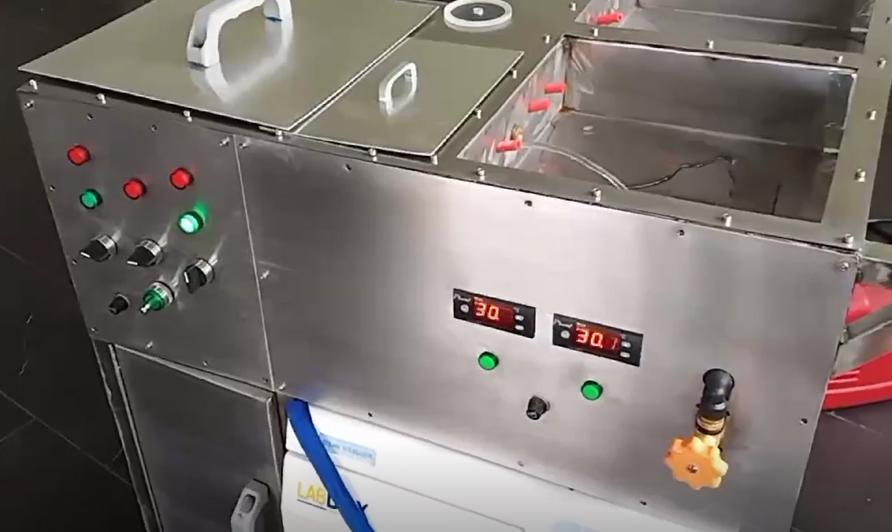
CleanIntubate is a device that facilitates automated cleansing, high-level disinfection, and sterilization of laryngoscope blades. The device is generally composed of: (1) a disinfection chamber that automatically dispenses detergent and disinfectant solutions, (2) a water sprinkler that dispenses water for rinsing, (3) a UV-C light bulb for sterilization, (4) a heater for drying, (5) a control box comprising a switch, a timer, and temperature control mechanism, and (6) a storage bin. Through this device, medical personnel are only required to rinse used laryngoscope blades in water until all visible soils are removed. The blades are then placed in the disinfection chamber where detergent and disinfectant solutions are automatically dispensed. Water, to rinse off the detergent and disinfectant solutions, is also automatically dispensed in the chamber. Once the water is drained, the heater is automatically activated to dry off the washed equipment. After drying, the UV-C light is activated to start the sterilization process. The drying temperature and time of the entire automated process can be set through the control box that is connected to the device.
This automated cleansing and disinfection process lessens the exposure of medical personnel to soiled or contaminated laryngoscope blades therefore also reducing their risk of contracting pathogens. The entire automated cleansing and disinfection process is also significantly faster compared to using regular washer-disinfectors.
Product Development Stage
CleanIntubate is at Technology Readiness Level (TRL) 4. The device is currently undergoing pre-clinical trials. A patent application for the device was already filed at IPOPHL through the funding assistance provided by PCHRD’s IPROTECH Program and through the assistance of DOST’s SciTech Superhighway Program. The technology generators intend to license the product to potential adopters after the completion of clinical trials.
Contact Person
Dr. Lourdes Marie S. Tejero
Director, UP Manila-Technology Transfer and Business Develop




