HeaRTNovation
Axis Revision Knee System
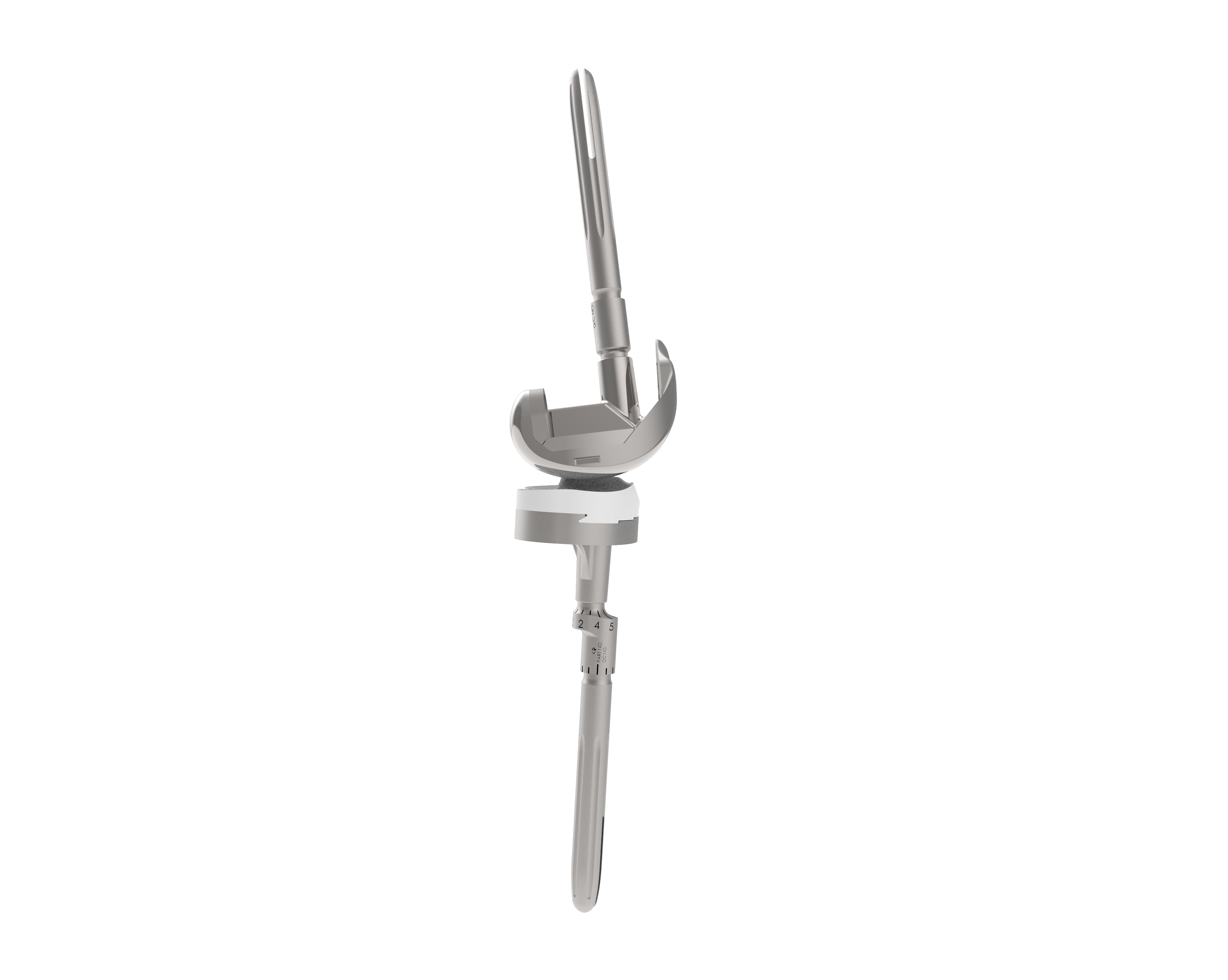
AXIS REVISION KNEE SYSTEM
Technology Generator
Orthopaedic International, Inc.
Project Leader: Ramon B. Gustilo, MD
The Problem
Total Knee Replacement (TKR) is a surgical procedure commonly done on arthritic patients who are experiencing chronic severe pain and impaired function of the knee joint. This is due to exhaustion of the articular cartilage that protects the bones from unnatural contact. It is estimated that around 600,000 patients undergo total knee replacement surgery per year in USA alone.
TKR is a highly successful procedure that significantly reduces pain, prevents disability, and enhances quality of life of orthopaedic patients. Despite TKR’s high success rate, the procedure is still prone to defects such as loosening of the components, infection and inflammation reaction, dislocation of the prosthetic joint, and other device complications. A revision TKR is necessary when failure of the primary TKR occurs. As this procedure is more expensive and complicated, there is a need for high-quality yet inexpensive revision implants.
The Solution
Axis Revision Knee System is a revision total knee replacement (TKR) system designed based on the Axis Knee System that was developed by the Orthopaedic International, Inc. (OII) through the funding support of DOST-PCHRD. Axis Knee System is the first world-class knee replacement system developed in the ASEAN Region. It uses a Femur Mechanical Axis Finder (FMAF) to allow surgeons to perform correct implant alignment by locating the true mechanical axis of the lower extremities. It is well-suitable for Asian knees, specifically for Filipinos, and is more accessible as it costs 50%-100% less than its competitors worldwide. As of March 2021, 779 Axis Knee Primary surgeries from all over the Philippines had already been performed.
Axis Revision Knee System is the solution developed to address complications of the primary TKR. It is composed of femoral, tibial, and patellar components that are made from metal and plastic materials. These high-quality implants come in a variety of sizes and are manufactured based on the standards of the American Society of Testings and Materials (ASTM) International.
With the support from DOST-PCHRD, the project team, composed of prominent surgeons and engineers from the Philippines, was able to simplify the surgical techniques and instrumentation of the Axis Revision Knee System using internationally-accepted procedures through a comprehensive research done from 2017 to 2019. Aside from the simplicity of the surgical procedures, the implants and the instruments are also manufactured locally. This significantly reduces the cost of knee revision arthroplasty making the system more accessible to many Filipinos who need the procedure.
Product Development Stage
The technology is currently at Technology Readiness Level 8. All validation studies of the technology are completed. Certifications of Product Registration for the thirteen (13) Axis revision knee implants as medical devices have been issued by the Food and Drug Administration (FDA). The system is ready for full commercial application.
Contact Details
Orthopaedic International, Inc.
No. 9 West Road, Light Industry and Science Park 1,
Economic Zone, Cabuyao Laguna, Philippines 4025
Telephone No.: (632) 885-7281 / 82, (6349) 543-0595
Fax: (632) 885-7280 / Email: info@oii.com
Website: https://www.oii.com




